-

-
Jawa-Huicheng made a sensational debut with its industry-first "Jawa·Traceability ProChain" digital production management solution at the 67th CIPM Exhibition.
16-18 Oct,2025
With the core concept of "serving the world from one platform", Jawa’s industry-first "Jawa·Traceability ProChain" not only meet the rigid global traceability compliance needs of enterprises but also facilitate the digital upgrade of production management. At the event, many industry partners and enterprise representatives stopped to conduct 1 to 1 consultation with Jawa’s consultant team. Through system practical demonstrations and explanations of real-world case outcomes, everyone more intuitively felt the new path that ProChain opens up for enterprise digital transformation and production efficiency improvement.
-

-
Jawa-Huicheng will attend the 67th CIPM (China International Pharmaceutical Machinery Expo) at Qingdao, 16-18 Oct, 2025
16-18 Oct,2025
The professional theme forum held at the same time as the Pharmaceutical Machinery Expo focuses on industry hot spots, industrial technological innovation, and the latest policies and regulations. Domestic and foreign industry experts and business leaders are invited to talk about cutting-edge trends, grasp industry trends, share practical experience, and assist the transformation and upgrading of the industry. and continued development.
The Expo site: QINGDAO COSMOPOLITAN EXPOSITION
Jawa-Huicheng theme forum: 14:00, Oct 2025, Conference Center -Room 207
Topic: New opportunities after the comprehensive application of drug traceability codes.
-
-
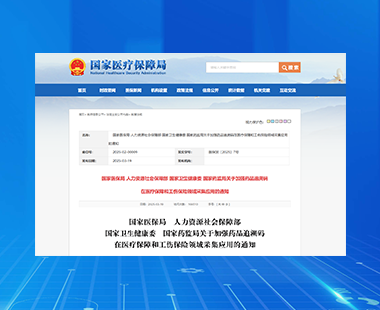
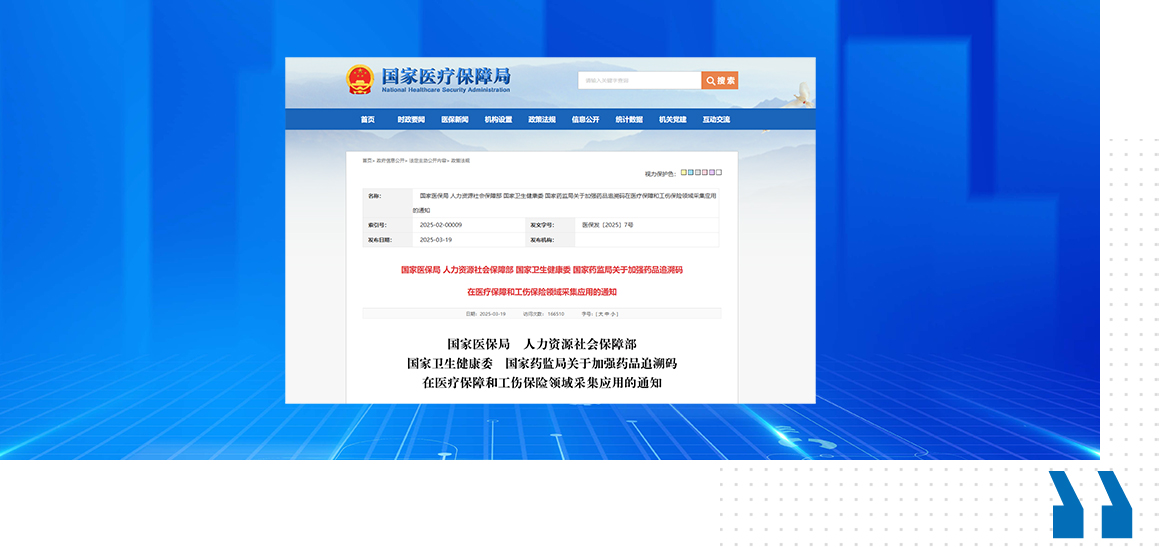
China’s National Healthcare Security Administration (NHSA) will tighten its regulation of medical insurance funds through a nationwide implementation of drug traceability codes in 2025.
1 July, 2025
In principle, starting July 1, 2025, medical insurance fund settlement will only be processed after the required code scanning is completed at the sales stage. Previously purchased drugs without traceability codes will be placed in a "no-code database" for management and temporarily eligible for medical insurance settlement. Starting January 1, 2026, all medical institutions will be required to collect and upload all drug traceability codes. The initiative aims to combat fraudulent activities such as the resale and substitution of insured medications, along with the misuse of medical insurance cards and counterfeit prescriptions.
-

-
Jawa-Huicheng made its overseas debut in Thailand ProPak Asia Exhibition, opening a new chapter of global cooperation.
14 June, 2025 ● Bankon, Thailand
On June 14, 2025, the 32nd Thailand International Packaging Exhibition (ProPak Asia), a four-day event, concluded successfully at the Bangkok International Trade Exhibition Center (BITEC). As a seasoned global service provider in traceability, Jawa Huicheng showcased its 'one item one code' traceability compliance and marketing digitalization solutions at an international exhibition. The company engaged in deep technical exchanges and cooperation discussions with industry experts, partners, and corporate representatives from over 40 countries and regions, marking a significant step towards expanding into overseas markets.
-

-
On April 25, the 66th CIPM concluded successfully!
25 April 2025 ● Chongqing, China
At this year’s pharmaceutical machinery exhibition, Jawa-huicheng made a wonderful appearance with innovative solutions such as industry traceability system construction plans and digital intelligence for production management, attracting many new and old friends to the scene.
-
-
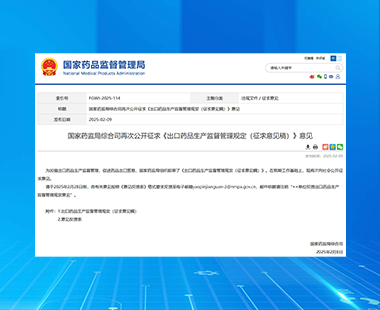
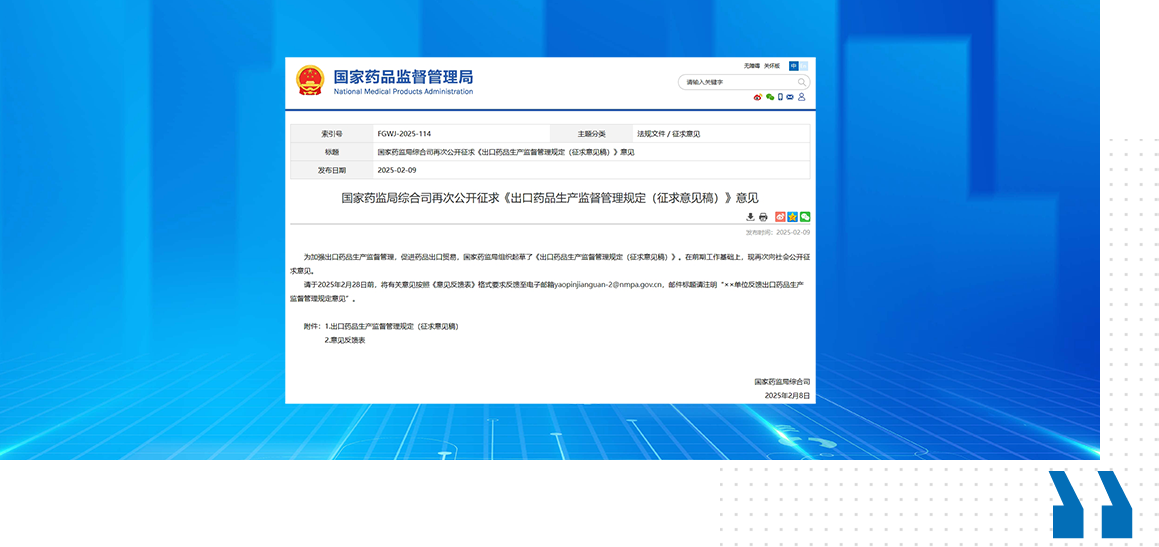
NMPA solicited opinions on the "Regulations on the Supervision and Administration of Export Drug Production (Draft for Comments)"
28 February, 2025
On February 9th 2025, the Comprehensive Department of the National Medical Products Administration once again publicly solicited opinions on the "Regulations on the Supervision and Administration of Export Drug Production (Draft for Comments)", aiming to strengthen the supervision and management of export drug production and promote drug export trade. Compared to the "Regulations on the Supervision and Administration of the Production of Exported Drugs (Draft for Comments)" released by the National Medical Products Administration on August 6, 2024, the newly released draft for comments has added chapters on "Entrusted Production of Exported Drugs" and "Export Drug Archives", and merged and adjusted some provisions.
The Management Regulations stipulate that if a pharmaceutical production enterprise accepts an entrusted agreement to produce and export drugs, it shall directly sign an entrusted production contract and quality agreement with the commissioning party. Both parties shall implement the drug management laws and regulations of China and importing countries (regions), as well as the quality responsibilities of drug GMP, and fulfill the obligation of drug quality assurance. The storage and transportation process of exported drugs within the country shall comply with the relevant requirements of the drug quality management standards for drug storage and transportation, ensuring that the entire process of drug export can be traced.
Source site:
https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjyp/20250209132616126.html?3jfdxVGGVXFo=1742458791594
-

-
China Launches National Pilot Program for Drug and Consumable Traceability Code Collection
30 September, 2024
In April of this year, the China National Healthcare Security Administration (NHSA) initiated a pilot program for collecting traceability codes for drugs and consumables covered by medical insurance. By the end of 2024, all 32 provincial-level medical insurance information platforms across China will fully implement the collection of these traceability codes.
On September 30th, the NHSA issued a notice aimed at further enhancing the collection of traceability code information for medical insurance drugs and consumables. The notice outlined that the NHSA is developing a unified interface for drug and consumable manufacturers and distributors to upload traceability code information at the national level. This new system will allow for a one-time upload that can be used across the entire country, eliminating the need for separate uploads to each province.
-

-
Jawa-Huicheng to Exhibit at the 64th CIPM in Qingdao, May 20-22, 2024.
23 May, 2024 ● Qingdao,China
Jawa-Huicheng was an invited exhibitor at the 64th CIPM, which is praised by exhibitors as the best pharmaceutical machinery exhibition in the Asia-Pacific region, on May 20-22, 2024, in Qingdao. The booth of Jawa-Huicheng was in the International hall; and Jawa-Huicheng showed and shared industry-leading medical track and trace system construction, UDI one-stop solution and results of global serialization practices. During CIPM, Jawa-Huicheng discussed requirements with many new and existing clients; who fully affirmed Jawa-Huicheng's capabilities.