


The supervision of medical cosmetic products faces numerous challenges, with many unlicensed and smuggled products present in the market. The root of this issue lies in the lack of unified standards for traceability systems, where various coding standards coexist, leading to incompatibility among different traceability systems. This chaotic market environment poses risks for both consumers and businesses, increasing the difficulty of regulation.
To address this situation, the China National Medical Products Administration (NMPA) officially issued the "Rules for the Unique Identification System of Medical Devices" in August 2019, marking the formal start of legislation to implement Unique Device Identification (UDI) in China. In the following two years, NMPA released two announcements on the effective implementation of UDI for the first and second batches of medical devices, clearly defining the scope, schedule, and work requirements for stakeholders. Additionally, to strengthen drug administration, NMPA introduced a "full traceability" mechanism and a drug recall system in 2019, and the new version of the Drug Administration Law proposed the establishment of a drug traceability system for the first time.

As a leading dermatology product enterprise, Galderma has 3 major groups of downstream distributors using various data exchange methods, some small distributors do not have an IT system.
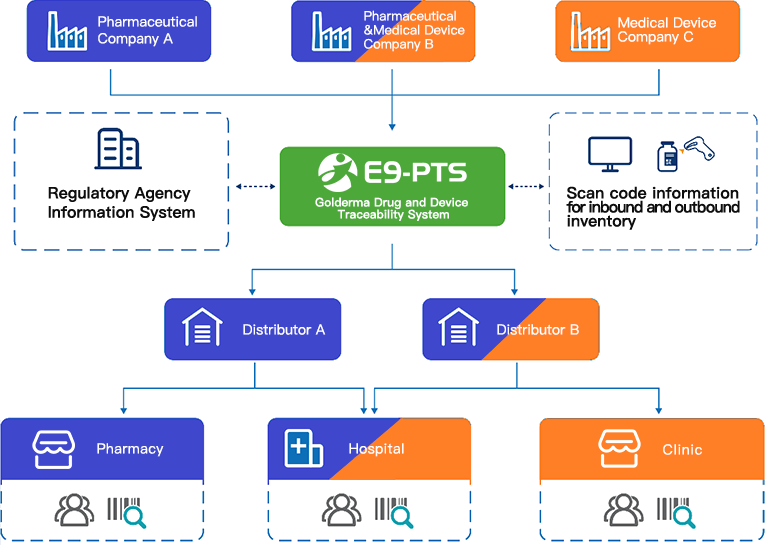
Currently, Golderma products on the market include medical devices which is based on UDI and contains serial numbers, medical cosmetics products tracked by production batch and managed as medical devices, and imported prescription drugs with DM code adopted GS1 standard. In the downstream distribution link, some distributors primarily sell medical device products, while others sell both prescription drugs and medical devices.Previously, due to the lack of a unified coding system, distributors managed sales, inventory, and flow data using multiple systems.

The "Jawa-Huicheng E9-PTS Traceability System" (hereinafter referred to as E9-PTS) is built based on the medical device UDI and drug traceability coding system, assisting Golderma and distributors in using a unified platform system and coding system to collect and trace the flow information of various products. The flow in the E9-PTS system is based on a one-item-one-code approach; for products managed only by batch, the system will provide batch inquiry reports to help users check the flow of entire batches of products. The system also supports GS1 EPCIS standards and can exchange data with traceability service platforms and regulatory systems in major countries.
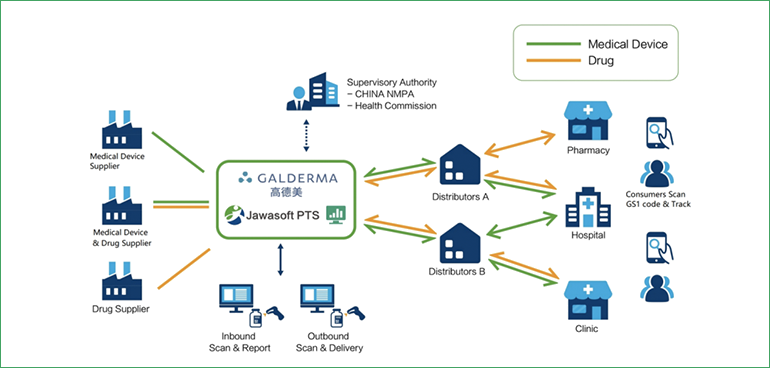
Based on the one-item-one-code approach and support from the E9-PTS system, Golderma products have achieved end-to-end traceability. For products managed only by batch, the E9-PTS system will provide batch inquiry reports to help users check the product flow. When Golderma distributors perform receiving tasks, they use PDAs or fixed devices equipped with the E9-PTS system to scan products or documents with GS1 barcodes, generating product receiving order numbers and completing the receipt process, while the system automatically updates inventory records. Similarly, when distributors ship products to downstream users such as clinics, they can automatically create outbound orders by using the E9-PTS program to scan GS1-128 one-dimensional barcodes containing GTIN or SSCC, as well as GS1 DataMatrix two-dimensional codes containing GTIN. Golderma's supply chain administrators can query traceability information for products and inventory in direct distribution warehouses through the PST system.
-

Jawa-Huicheng has initially implemented a traceability system based on GS1 international standards that allows for the simultaneous tracking of pharmaceuticals and medical devices. In the future, this system can help companies meet regulatory requirements for drug traceability and unique identification of medical devices using the same standard and system, ensuring safety for patients using medications and devices. Additionally, it can facilitate the digitalization of the supply chain and quality control for distribution enterprises, as well as provide data and system support for pharmacies and medical institutions to achieve refined management.