

As early as 2015, Qilu Pharmaceutical Group and Jawa-Huicheng successfully established a DSCSA compliance traceability system, setting a precedent
for the operation of serialization systems in Chinese factories.
Jawa's traceability products and services comprehensively support Qilu Group's diverse business segments, including both domestic and export
operations. As a result, Jawa has emerged as a strategic service provider for Qilu Pharmaceutical's export drug serialization.

As of the end of 2023, Qilu Group's pharmaceutical products have been exported to over 90 countries and regions worldwide, with approximately
40 products having the highest market share globally or in the countries where they are sold, benefiting approximately 1 billion patients worldwide.
Jawa’s serialization & traceability system provides key values to Qilu Pharma:
• Capable of automatically handling multiple varieties, specifications, and packaging formats of products on the same production
line, ensuring high efficiency for both large-scale continuous production and small batch orders.
• Leverages existing equipment to enable quick upgrades, transformations, and implementations of the serialization system for
workshop production lines.
• Jawa's "one-stop service" enables all units of Qilu to concentrate on their core business, ensuring seamless drug traceability
and serialization.
Jawa’s NT-Track serialization platform is the L4 part of the manufacturer traceability system, responsible for important functions such as connection,
collaboration, and product lifecycle traceability management.

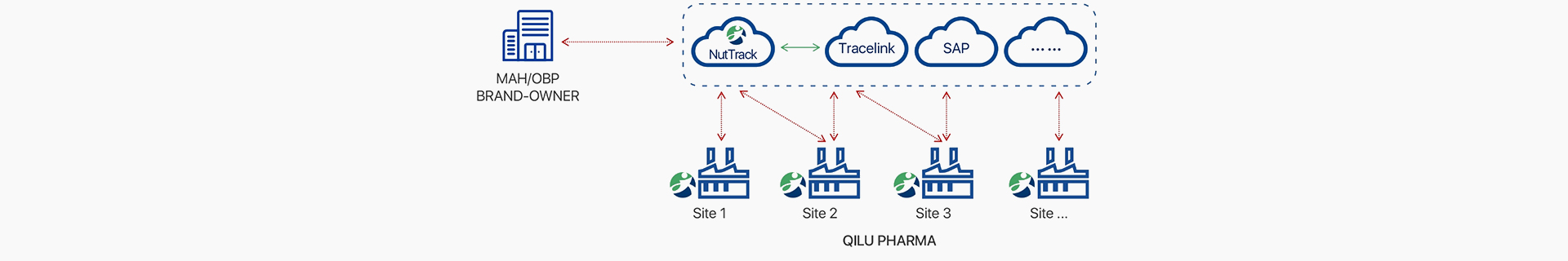
The Jawa system efficiently manages various processing factories within the enterprise. It allows for the allocation of serial numbers (SN) to different
factories based on production plans. After production is completed, the production data can be uploaded to the L4 system through the platform's interface.
The platform supports data uploads for various EPCIS events and is compatible with communication protocols such as HTTPS, SOAP, AS2, and SFTP.
Additionally, the system operates in silent management mode and features plugin publishing capabilities.
The main functions of Jawa serialization system and export drug solutions:
• Workshop management
• Master-data maintenance (trading partners, brand-owners...)
• Production management (product and task management)
• Serial number request and provision
• EPCIS Events data upload (commission, aggregation, shipping events)
• Barcode generator template
• Order management (Outbound order upload, inbound management, etc.)
• Sampling management
Jawa expert team provides consultation services to help Qilu achieve low-cost and efficient integration with L4 platforms for multinational clients and wholesalers.






